* Life * Research * Experiments * Media < back
How Otto Hahn researched and gained his knowledge
Otto Hahn's field of activity was nuclear research. He was thus dealing with an object so small he could not see it with the naked eye, or even the best magnifying glass. Atoms were thought of at that time as the basic particles which composed everything around us in the world, and could not be split further. For his work, Hahn was able to draw on important insights from colleagues who had already developed models to even imagine atoms.
According to these models, each atom consisted of a shell and a nucleus, with electrons forming the shell. These are negatively charged and move in different orbits around the atomic nucleus, consisting of protons that are positively charged, as well as neutrons. These neutrons are the buffer, so to speak, between protons that would otherwise be repelled by their identical charge. The number of protons determines the properties of a substance or element. Atoms with different substances combine to form molecules and create new elements with new properties.

Otto Hahn's special research focused on so-called radiochemistry. He dealt with radioactive elements, for example uranium, and was lucky enough to be able to work in very well-equipped laboratories. He also had colleagues who were great specialists in the field of radioactivity research, especially Lise Meitner. Thus he was able to make certain assumptions, develop appropriate experiments, and see his preliminary results confirmed in further experiments, or not. He could never see directly what had actually happened, but only gain insights through the effects of what must have happened.
In this way, Hahn succeeded in identifying new elements or subspecies of an element (isotopes), which are created by a process that can be explained as follows: The atoms of radioactive elements contain many more neutrons than protons in their core. As a result, these nuclei are less stable and decay into new elements or isotopes. It is in this context that Hahn succeeded in the experiment that made him world famous. He irradiated the radioactive, unstable nuclei of atoms with additional neutrons, splitting the nucleus into two nuclei with their impact and releasing further neutrons - which in turn split other nuclei, and so on.
Only after the experiment, and long discussions with colleagues and the physicist Lise Meitner, did it become clear what must have happened, and what Otto Hahn had first succeed in doing: it was actually possible using certain methods to split atoms, which had been considered indivisible. This meant atoms couldn't be the smallest existing particles. It was the first time nuclear fission had been recognized as such, and theoretically explained. Since the process of nuclear fission releases large amounts of energy, which could be used peacefully or for military purposes, it marked the beginning of intensive research.
How Otto Hahn came to his research subject
As a teenager, Otto Hahn had already performed “chemical gimmicks” as he later called them, in the laundry room at home. Some of these activities were not entirely safe: He experimented with hydrogen, sodium or with potassium chlorate and phosphorus. School was likely not particularly interesting for Otto - especially the physics and chemistry lessons he found rather boring and dry.
Since he was very interested in the structure, properties and conversion of substances, it was only natural that he should study chemistry after high school, among other subjects. Although during his studies Hahn had little contact with his later research subject, when he was employed at the University College in London and became an assistant to Sir William Ramsay (1852-1916), he was told: "You will work on radioactivity". It was the field of research he would become an outstanding scientist in.
His physicist colleague Lise Meitner said about him: “Hahn is one of the founders of radiochemistry, and has discovered a significant number of new radioactive substances. He understood with great ingenuity how to apply radiochemistry to a wide range of physical, chemical and geological problems. Ultimately, his greatest achievement, the discovery of the uranium fission for which he received the Nobel Prize, belongs in this field of activity.
It came about through the unusually good quality of chemistry research by Hahn and Straßmann - fantastically good chemistry work with no equals at that time. The Americans learned it later, but at that time Hahn and Straßmann were really the only ones who could even do it, because they were such good chemists. They in fact demonstrated a physical process through chemistry, so to speak. ”
How Otto Hahn documented his findings
Otto Hahn published many books in which he wrote about his research, such as the conversion of the atomic nuclei; many been translated into several languages.
Especially after the Second World War, Otto Hahn was heard on the radio in interviews and lectures. In addition to explaining his scientific work, he mainly campaigned for the peaceful use of nuclear power and the ban on nuclear weapons. The documentation created during his research work is preserved in the archive of the Max Planck Society.
How Otto Hahn's findings were advanced and the importance of his research today
 At the start of Otto Hahn's experiments with radioactive elements, it was not clear whether and how his findings could be used. But after he succeeded in splitting atomic nuclei in 1938, an extremely rapid development for the technical use of his research results began. The first atomic bombs were dropped in Japan just seven years after Hahn's discovery, and had a destructive effect previously unknown to mankind.
At the start of Otto Hahn's experiments with radioactive elements, it was not clear whether and how his findings could be used. But after he succeeded in splitting atomic nuclei in 1938, an extremely rapid development for the technical use of his research results began. The first atomic bombs were dropped in Japan just seven years after Hahn's discovery, and had a destructive effect previously unknown to mankind.
After another nine years, in 1954, the first nuclear power plant in the Soviet Union went into operation. A year later a nuclear power plant in England began to produce electricity.
Although Otto Hahn fought against the development and spread of nuclear weapons, he viewed the peaceful use of nuclear energy as positive. Following the serious accidents at the nuclear power plants in Harrisburg, Chernobyl and Fukushima, we now know how dangerous this type of energy generation is. Nevertheless, new nuclear power plants are still being built to generate electricity. And despite the many disarmament treaties that exist today, there are still enough nuclear weapons to destroy all of humanity.
Since Hahn's work with radioactive elements, there have been not only great technical advancements, but also new scientific insights in atomic research. Before his findings, it was assumed an atomic nucleus could not be split. After Hahn had succeeded in this, it was then believed that electrons, protons and neutrons were no longer divisible. Today it is assumed that electrons, protons and neutrons are also composed of even smaller particles: protons and neutrons consist of so-called quarks.
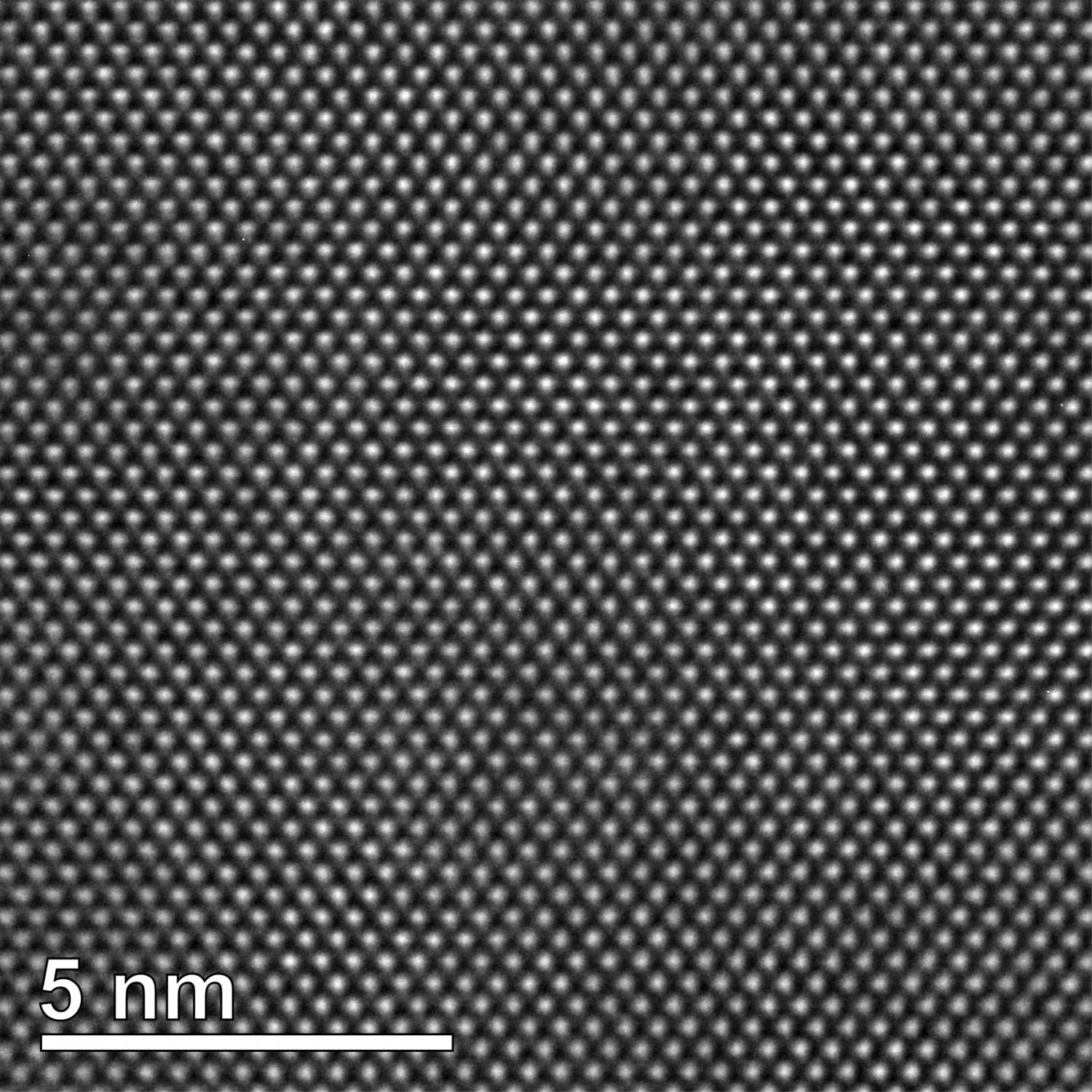
The perception of the smallest particles that make up all matter is therefore constantly changing. This of course also changes the models designed to assist in imagining the structure of atoms. Today some of the structures that form atoms can actually be seen with microscopes. The regular three-dimensional lattice, for example, to which the atoms are arranged in crystals, can be imaged in a transmission electron microscope (TEM).

Aside from the scientific and technical developments in atomic research, another problem remains unsolved to this day: whether scientists are responsible for the consequences of their research. Otto Hahn felt responsible when US planes dropped atomic bombs in August 1945 on the Japanese cities of Hiroshima and Nagasaki, killing more than 300,000 people, and he always spoke publicly about it.
In his acceptance speech at the awarding of the Nobel Prize, Hahn warned of the spread and further development of nuclear weapons. Together with other scientists who had worked together on the atomic project of the 1940s, he signed the Göttingen "Declaration of 18 Atomic Scientists" in 1957, speaking out against the arming of the Bundeswehr with nuclear weapons.
* Life * Research * Experiments * Media < back